

So this would be MV isĮqual to MV, and let's do the molarity of the base Let's see what happens if you try to use MV is equal to MV, that shortcut that we learned about in the last video. Of HCl is equal to 0.0422 molar, and we are finally done, right? That's our concentration So we can take 0.000844, and we can divide that by 0.0200, and we get for our answer here 0.0422 molar. That was 0.0200 liters, right? 20 milliliters is equal to 0.0200 liters. All right, so the concentration of HCl in our original solution would be, we had 0.000844 moles. So now we have moles, right? We have moles, and we have liters. Right, and 20 milliliters would be, move our decimal place, 0.0200 liters. Right, so we started withĢ0 milliliters of HCl. We just have to calculate the concentration of ourĪcid solution, right? Let's go back up here so weĬan see what we started with. And so we get X is equal to 0.000844, right? That's how many moles of HCl we have at our equivalence points. Right, and our moles of barium hydroxide, let me go ahead and useĪ different color here.
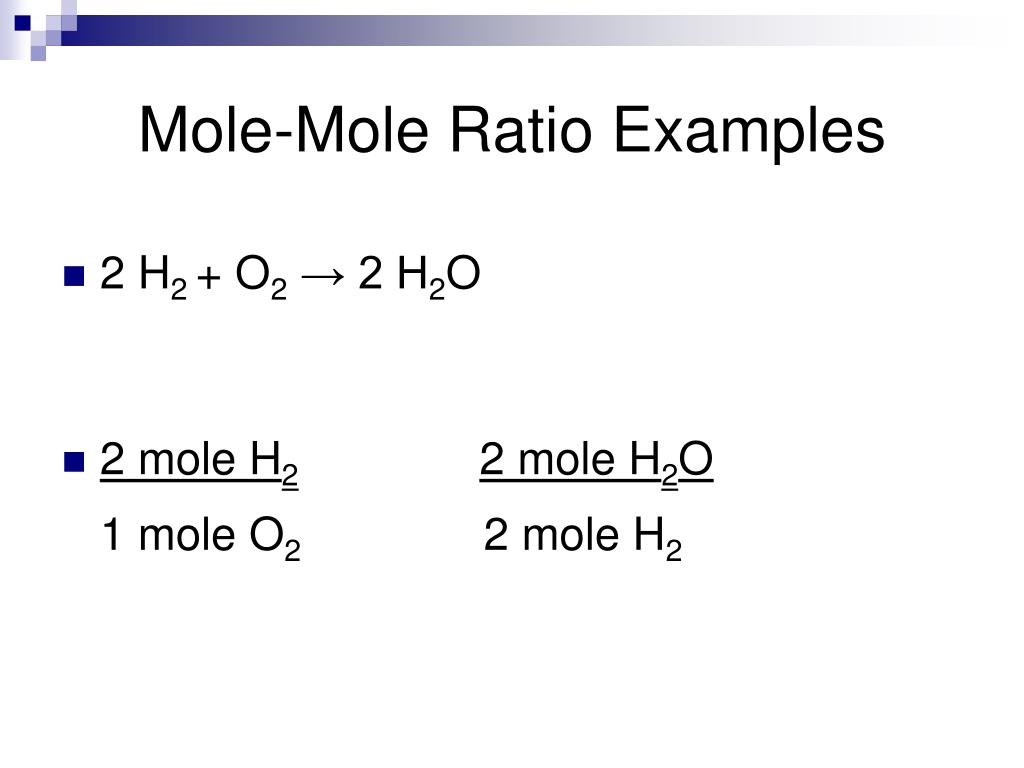
Talking about a ratio of barium hydroxide to HCl, our mole ratio is one to two. So we can multiply this number by two, and we'd figure out how So therefore, we had twice as many of HCl. Many moles of barium hydroxide that we used in our titration, right? That's 0.000422. So for every one mole of barium hydroxide, we have two moles of HCl. Let's look at the mole ratioįor barium hydroxide to HCl. Should be balanced, right? Everything else should be balanced. Here for this coefficient to give us four hydrogens on the right. On the right, we have only two hydrogens. Have two hydrogens here, and then we have two over here. So we need to put a two right here, and now we have twoĬhlorines on both sides. All right, next we need toīalance our equation, right? We need to balance the So BACl2, right? So BACl2, barium chloride,Īs our other product here.
Chemlab 12 a mole ratio plus#
So we have BA2 plus and CL minus 1, so you could cross those over. This is barium two plus, right? This is BA2 plus, and over Gives us, for our products, we have H plus and OH The negative fourth, right? So that's equal to 0.000422 moles of barium hydroxide. So we take 0.0154 and we multiply that by 0.0274, and that gives us, this will be 4.22 times 10 to We solve for X, and X of course represents the moles of barium hydroxide. 27.4 milliliters is 0.0274 liters, right? So that's 0.0274 liters. All right, so we have 0.0154 as equal to, let's make moles X, over liters. It's 0.0154 molar, and we also know that molarity is equal to moles over liters. Of barium hydroxide to completely neutralize Takes 27.4 milliliters of a 0.0154 molar solution Hydroxide, we're going to use barium hydroxide, and it Of HCl, and this time, instead of using sodium Another titration problem, and once again, our goal is to find the concentration


 0 kommentar(er)
0 kommentar(er)
